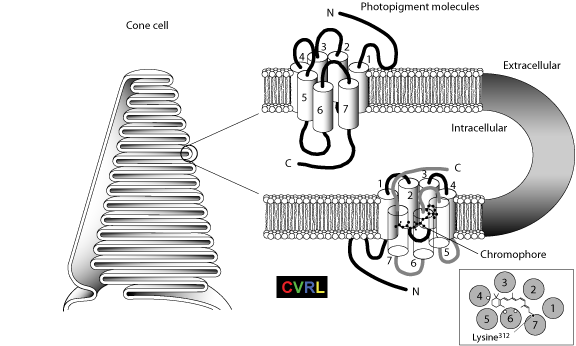

Figure 1.2 from Sharpe, L. T., Stockman, A., Jägle, H., & Nathans, J. (1999). Opsin genes, cone photopigments, color vision and colorblindness. In K. Gegenfurtner & L. T. Sharpe (Eds.), Color vision: From Genes to Perception (pp. 3-51) Cambridge: Cambridge University Press.
Cutaway view of the photopigment molecules (right) packing within the enfolded membrane discs in the outer segments of the cone photoreceptor cells (left). Each molecule consists of a transmembrane opsin bound to a chromophore, 11-cis-retinal. The opsin or protein portion of the molecule is a chain of amino acids, running from an amino-terminal end (N), exposed on the external aqueous surface of the membrane discs, to a carboxyl terminal region (C), exposed on the internal aqueous surface of the discs. The chain has seven coils, termed α-helices, spanning the membrane (Hargrave et al., 1984). Linked together by loops in the rest of the chain, the α-helices encircle the chromophore (right, lower cutaway view). The loops are distinguished by whether they occur in the luminal (extracellular) or cytoplasmic (intracellular) face of the cell. The view is from the intracellular surface. It indicates the approximate position of the a-helices and of the three amino acids (open circles) that are believed to have the major influence on the lmax of the pigment (see Fig. 1.5B). The tail of the chromophore is attached by a protonated Schiff base to a charged lysine amino acid residue lying at nucleotide position 312 in the chain of the L- and M-cone opsins (filled circle, see Fig. 1.5B) and at position 293 in the chain of the S-cone opsin (corresponding to position 296 in rhodopsin). Features critical to the function of the opsin are well conserved in all known mammalian species, with the interhelical loops being, on average, more conserved than the transmembrane helical regions.
Curcio, C. A., Allen, K. A., Sloan, K. R., Lerea, C. L., Hurley, J. B., Klock, I. B., et al. (1991). Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. Journal of Comparative Neurology, 312, 610-624.
Curcio, C. A., & Sloan, K. R. (1992). Packing geometry of human cone photoreceptors: variation with eccentricity and evidence for local anisotropy. Visual Neuroscience, 9, 169-180.
Sharpe, L. T., Stockman, A., Jägle, H., & Nathans, J. (1999). Opsin genes, cone photopigments, color vision and colorblindness. In K. Gegenfurtner & L. T. Sharpe (Eds.), Color vision: From Genes to Perception (pp. 3-51) Cambridge: Cambridge University Press.
Stockman, A., & Sharpe, L. T. (2000). Spectral sensitivities of the middle- and long-wavelength sensitive cones derived from measurements in observers of known genotype. Vision Research, 40, 1711-1737.